FUTURE
Many CA enzymes have been identified and characterised recently (Faridi & Satyanarayana, 2016; Hou et al., 2019; Li et al., 2015). However, when immobilised on magnetite nanoparticles, proper kinetic data for these enzymes is needed. The first step was establishing a set of procedures for producing enzymes immobilised on cMNPs, which the team had accomplished. With these established protocols, we aim to produce different origin CA enzymes using the modified plasmid and its incorporation into Pichia pastoris and study their activity once immobilised on our cMNPs. Upon optimisation of the process, we plan to implement these nanoparticles in biomass production (Yuan et al., 2022).
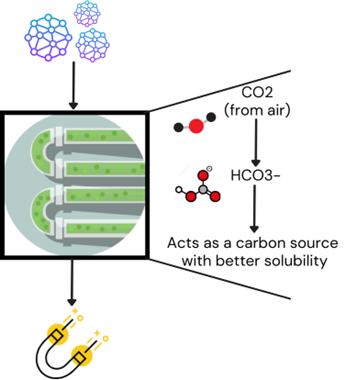
Figure 1: Use of nanoparticles in optimization of biomass production
Why biomass? The search for alternative energy sources is ongoing with depleting fossil fuel reserves. The thermal treatment of biomass through gasification, hydro treatment, and pyrolysis generates bio-oil, biofuels, bio-char, and biogas, which can help address the energy crisis (Kumar et al., 2020). Different microbes can be used as a source of biomass, such as bacteria, higher fungi, and microalgae. For example, microalgae are a potential source of lipids which, after further processing, can be converted into biodiesel (Wang et al., 2024). Another research showed that fungi like P. chlamydosporia can convert chitosan to ethanol (Aranda-Martinez et al., 2017). Cyanobacteria is also a promising source for synthesising biofuels and biodegradable plastics (Agarwal et al., 2022).
Using CA-functionalized magnetite nanoparticles can help overcome the mass transfer resistance due to the low solubility of CO₂, simplifying downstream processing. The conversion of CO₂ to H₂CO3 in the presence of water increases overall solubility, enhancing the growth rate of photosynthetic microbes for biomass production. We aim to test these nanoparticles to enhance the growth rate of different microorganisms, which can be used as a valuable source of biomass.
Another interesting question: how can we integrate these nanoparticles into the bioreactor system? While designing a reactor, many parameters must be accounted for. The rate of air supply, temperature and pH control, nutrient composition, impeller speed for culture mixing, foam control and material of construction are some of the essential features of a bioreactor. We directly added the immobilised nanoparticles to the culture at the lab scale. In a bioreactor, these immobilised nanoparticles can be directly added or incorporated into structures like polymeric beads, magnetic clusters, micelles, etc. (Vaghari et al., 2016).
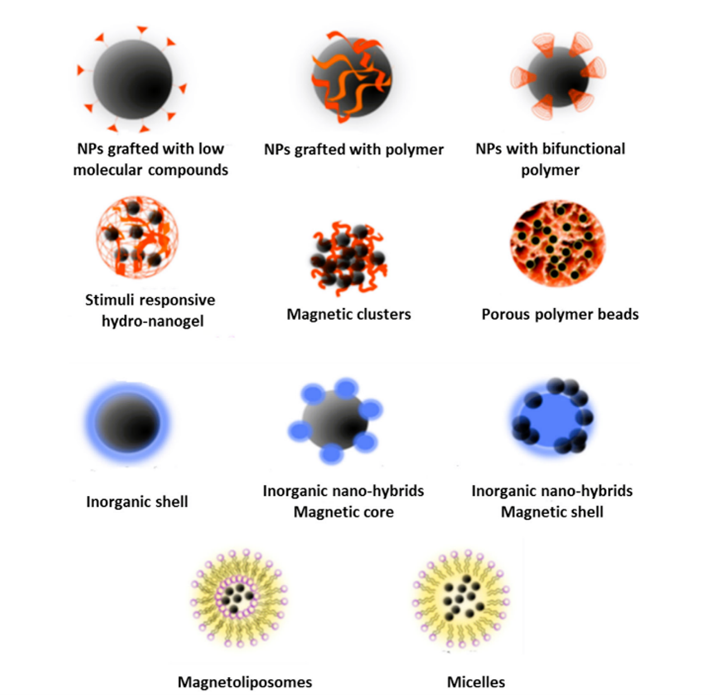
Figure 2: Different structures/supports for nanoparticles (Vaghari et al., 2016)

Figure 3: PBRs in ICT, Mumbai
Photobioreactors (PBRs) are gaining popularity in cultivating photosynthetic microorganisms because they provide better control, improved growing conditions for photosynthetic microbes, and enhanced biomass yields. One of the significant applications of PBRs is in CO₂ capture. Incorporating structured supports in the zones where light effectively penetrates PBRs can improve the growth rate of microbes. However, the scale-up of PBRs is still in the works (Sirohi et al., 2022). In conclusion, this project is not about capturing carbon but converting it into a more valuable product than a diamond.
Here, we provide a detailed analysis of the scale-up of this project. Please note that the following costs may vary depending on availability and the region of purchase.
1. Scalability of the Experiment:
-
a. Recombinant CA Production in Pichia pastoris:
- i. Industrial Feasibility: Using P. pastoris allows for high-yield expression of the CA enzyme with post-translational modifications essential for functionality. This yeast can grow to high cell densities in bioreactors, making it an ideal host for large-scale production.
- ii. Cost Efficiency: Once the genetic transformation is complete, P. pastoris can produce CA with minimal setup changes, significantly reducing production costs compared to continuous plant-based extraction.
- iii. Reproducibility: This method ensures consistent enzyme quality and quantity across batches, which is critical for scalability.
-
b. Plant-Based Extraction:
- i. Challenges in Scale-Up: The extraction from spinach involves labour-intensive steps like washing, blending, centrifugation, and dialysis. Spinach quality variation could impact enzyme yield and activity, limiting its scalability.
- ii. Raw Material Sourcing: Ensuring a continuous supply of fresh spinach at an industrial scale could add logistical challenges and costs.
- iii. Suitability: This method may be more suited for laboratory or small-scale applications where fresh plant material is easily accessible.
-
c. Magnetite Nanoparticle synthesis:
-
i. Advantages of Coprecipitation Method:
- 1. Simplicity and Efficiency: The method’s simplicity makes it well-suited for scale-up, as the core process of mixing, reaction, and separation can be easily adapted for larger volumes.
- 2. Consistent Particle Quality: By controlling reaction conditions, particle size and magnetic properties are kept uniform, essential for industrial-scale consistency.
- 3. Low Energy and Cost: The process operates at ambient or slightly elevated temperatures, reducing energy requirements compared to high-temperature methods.
-
ii. Challenges in Scaling:
- 1. Reaction Environment Control: Scaling up would require maintaining an inert atmosphere to prevent oxidation. This would necessitate a closed or controlled reactor system, especially for large-scale production.
- 2. Magnetic Separation Logistics: At a larger scale, magnetic separation of nanoparticles from reaction mixtures would require stronger magnets and possibly multi-stage separation to ensure efficiency and minimise loss.
- 3. Waste Management: The need for repeated washing steps using deionised water could generate significant wastewater at an industrial scale. Efficient recycling and waste management systems would be essential to keep the process cost-effective and sustainable.
-
i. Advantages of Coprecipitation Method:
-
d. Conclusion:
- The recombinant production method in Pichia pastoris is considerably more scalable and industrially viable than plant-based extraction. Furthermore, the coprecipitation method is inherently scalable, with some adaptations required for inert gas maintenance, large-scale magnetic separation, and efficient water use management.
2. Cost and Economics of the Process:
-
a. Recombinant Method:
- i. Initial Investment: Genetic engineering and transformation require specialised equipment and reagents (e.g., plasmids, restriction enzymes, selective media), which might be costly initially. Since all the equipment was in our institute, we did not include them above. We suggest that the industry build an on-site biotechnology lab. The initial investment can vary from Rs 50 Lakhs to Rs 1 Crore. This minimises the risk of damage or loss in transporting recombinant P. pastoris from different locations. Many of the chemicals, buffers, and reagents were available in the lab, due to which our budget decreased substantially. The best approximation of the cost required is up to Rs 3 Lakhs.
- ii. Economic Long-Term Production: After the initial setup, this method becomes cost-efficient. With P. pastoris, high enzyme yields reduce the cost per unit of CA produced, making it viable for industrial CO₂ sequestration.
- iii. Reduced Chemical Dependency: This approach avoids recurring expenses for plant-based reagents like ammonium sulphate, making it more sustainable.
-
b. Plant-Based Extraction:
- i. Variable Cost: Dependence on fresh spinach for raw materials introduces variability in cost. Storage, handling, and extraction of enzymes require consistent replenishment of chemicals and buffers, impacting the cost-effectiveness. At the lab scale, procuring the raw material is cheap as it is available in any vegetable market. Procuring the same at the industrial level is difficult. The equipment (like dialysis membrane, centrifuge, stirrer) can cost around Rs 50 thousand to 2 Lakh. Even though fewer reagents are required initially, it will be counted as a recurring expense (up to Rs 50 thousand). The total amount can double or triple depending on the enzyme's production scale.
- ii. Labor and Equipment: This method requires extensive manual processing steps, which may increase labour costs and limit economic efficiency.
-
c. Magnetite Nanoparticle synthesis:
-
i. Materials Cost:
- 1. Iron Salts and NaOH: The primary reagents (iron salts and ammonium hydroxide) are relatively inexpensive and widely available, making them cost-effective at scale. We bought 500 gm ammonium sulphate lab grade at Rs 360. The cost is significantly reduced if the chemical is ordered directly from the manufacturer in bulk. The same applies to the order of ferrous salts.
- 2. Inert Atmosphere: Using an inert gas like nitrogen could add to operational costs, especially in larger-scale reactors, but closed systems or recirculating inert gases could mitigate this. Commercial-grade Nitrogen gas costs Rs 90/ cubic metre. Nitrogen storage in low-pressure cylinders will cost around Rs 1 to 2 Lakhs per cylinder.
-
ii. Equipment and Setup:
- 1. Reactor Requirements: At larger scales, reactors with effective agitation and precise temperature/pH control will be necessary to maintain consistent particle quality. Manufacturing and construction costs are dominant. This may vary from vendor to vendor. It could range from Rs 5 Lakhs to 5 Crores.
- 2. Magnetic Separation: Enhanced magnetic separation equipment will be needed as volume increases. Scalable magnetic separators exist but would be a considerable investment if consistently high yield and purity are desired. Starting prices of small-sized magnetic separators are Rs 2 Lakhs.
-
i. Materials Cost:
-
d. Conclusion:
- While initial costs for recombinant production are high, the long-term cost savings and consistency in enzyme output make it more economically viable than the plant extraction method. The economic feasibility of the coprecipitation method process at scale is favourable due to low-cost reagents and manageable equipment requirements. However, investment in inert gas control and advanced separation systems is necessary.
-
e. Comparing Alternative Methods of MNP Synthesis:
- The coprecipitation method has advantages over other MNP synthesis methods, particularly for scalability:
- i. Thermal Decomposition: This alternative method offers excellent control over particle size and morphology but requires higher temperatures and organic solvents, which are more costly and less environmentally friendly at scale.
- ii. Solvothermal Synthesis: Although highly effective, this method also relies on high-pressure systems, which are complex to scale up and costly in terms of energy and equipment.
Hence, the coprecipitation method is favourable for large-scale operations, balancing cost, simplicity, and control over particle quality, making it better suited for industrial applications.
3. Activity of Enzyme Expected:
-
a. Recombinant Production:
For our future experiments, we will screen more CA genes, which will be more stable.
- i. High Stability and Activity: The yeast-based enzyme production is expected to yield a highly active enzyme with consistent performance.
- ii. Improved Immobilization: Immobilizing CA on carboxyl-functionalized MNPs enhances stability and reusability. This approach demonstrates improved catalytic activity for CO₂ conversion in controlled settings, making it suitable for sustained sequestration.
-
b. Plant-Based Extraction:
- i. Lower Stability: CA extracted from spinach has lower thermal and pH stability, affecting its suitability for CO₂ sequestration processes involving varied environmental conditions.
- ii. Limited Reuse Potential: Immobilization might improve its utility somewhat, but variability in the enzyme's initial activity could limit efficiency.
-
c. Conclusion:
- Recombinant CA from P. pastoris is expected to deliver higher, more consistent enzyme activity suitable for industrial applications.
4. CO₂ Capture Results and Efficacy Analysis:
The documents indicate CO₂ capture efficacy was tested using CA immobilised on MNPs. Growth curve analyses with E. coli cultures showed enhanced CO₂ utilisation when CA-MNPs were introduced, increasing cell density, and demonstrating the enzyme's catalytic efficiency in converting CO₂. This immobilised system shows promise for scalability, with a stable enzyme system that could maintain activity across multiple cycles in industrial applications. Using magnetite nanoparticles also supports easy recovery and reuse of the immobilised enzyme, reducing operational costs in CO₂ sequestration processes.
Conclusion and Recommendations: The recombinant production of carbonic anhydrase in P. pastoris and immobilisation on magnetite nanoparticles produced by the coprecipitation method presents a scalable, cost-effective solution for industrial CO₂ sequestration. This approach is preferable over plant extraction due to its reproducibility, high enzyme yield, stability, and cost-effectiveness for long-term applications.
Recommendations for Further Development:
- Pilot Scale Testing: Conduct pilot-scale tests using bioreactors to assess scalability and optimise fermentation conditions. Testing the coprecipitation process in a controlled reactor environment at an intermediate scale would help optimize inert gas usage, stirring, and magnetic separation on a larger scale.
- Economic Analysis: Detailed cost-benefit analysis comparing recombinant and plant-based methods in various production scales.
- Operational Stability Testing: Investigate CA-MNP stability across various operational conditions to refine its industrial application in CO₂ sequestration.
- Magnetic Separation Optimization: Multi-stage magnetic separation and advanced magnetic separators should be explored to enhance recovery efficiency at scale.
- Wastewater Treatment: Develop efficient water recycling and treatment protocols to minimize costs and environmental impact from washing steps.
References
Agarwal, P., Soni, R., Kaur, P., Madan, A., Mishra, R., Pandey, J., Singh, S., & Singh, G. (2022). Cyanobacteria as a Promising Alternative for Sustainable Environment: Synthesis of Biofuel and Biodegradable Plastics. Frontiers in Microbiology, 13, 939347. https://doi.org/10.3389/fmicb.2022.939347
Aranda-Martinez, A., Naranjo Ortiz, M. Á., Abihssira García, I. S., Zavala-Gonzalez, E. A., & Lopez-Llorca, L. V. (2017). Ethanol production from chitosan by the nematophagous fungus Pochonia chlamydosporia and the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Microbiological Research, 204, 30–39. https://doi.org/10.1016/j.micres.2017.07.009
Faridi, S., & Satyanarayana, T. (2016). Novel alkalistable α-carbonic anhydrase from the polyextremophilic bacterium Bacillus halodurans: characteristics and applicability in flue gas CO₂ sequestration. Environmental Science and Pollution Research, 23(15), 15236–15249. https://doi.org/10.1007/s11356-016-6642-0
Hou, J., Li, X., Kaczmarek, M. B., Chen, P., Li, K., Jin, P., Liang, Y., & Daroch, M. (2019). Accelerated CO₂ Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. International Journal of Molecular Sciences, 20(6), 1494. https://doi.org/10.3390/ijms20061494
Kumar, A., Bhattacharya, T., Mozammil Hasnain, S. M., Kumar Nayak, A., & Hasnain, M. S. (2020). Applications of biomass-derived materials for energy production, conversion, and storage. Materials Science for Energy Technologies, 3, 905–920. https://doi.org/10.1016/j.mset.2020.10.012
Li, C.-X., Jiang, X.-C., Qiu, Y.-J., & Xu, J.-H. (2015). Identification of a new thermostable and alkali-tolerant α-carbonic anhydrase from Lactobacillus delbrueckii as a biocatalyst for CO₂ biomineralization. Bioresources and Bioprocessing, 2(1), 44. https://doi.org/10.1186/s40643-015-0074-4
Yuan, Y., Wang, F., Li, H., Su, S., Gao, H., Han, X., & Ren, S. (2022). Potential application of the immobilization of carbonic anhydrase based on metal organic framework supports. Process Biochemistry, 122, 214–223. https://doi.org/10.1016/j.procbio.2022.10.019
Wang, M., Ye, X., Bi, H., & Shen, Z. (2024). Microalgae biofuels: illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnology for Biofuels and Bioproducts, 17(1), 10. https://doi.org/10.1186/s13068-024-02461-0
Vaghari, H., Jafarizadeh-Malmiri, H., Mohammadlou, M., Berenjian, A., Anarjan, N., Jafari, N., & Nasiri, S. (2016). Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnology Letters, 38(2), 223–233. https://doi.org/10.1007/s10529-015-1977-z